A Non-Flammable Potassium-ion Battery
Rechargeable batteries based on potassium and potassium-ion could be a promising and cheaper alternative to Lithium. Safety issues have dogged the development of a battery based on these materials because non-flammable electrolytes have so far not been developed...
Rechargeable batteries based on potassium and potassium-ion could be a promising and cheaper alternative to Lithium. Safety issues have dogged the development of a battery based on these materials because non-flammable electrolytes have so far not been developed.
Researchers from the University of Wollongong (Australia) and the Shanghai University of Engineering Science (China) have been working on a non-flammable electrolyte for potassium and potassium ion batteries. The new electrolyte is based on an organic phosphate which is effective even at the relatively low concentration levels necessary for mass production processes.
The research findings are published in the journal Angewandte Chemie.
Researchers from the University of Wollongong (Australia) and the Shanghai University of Engineering Science (China) have been working on a non-flammable electrolyte for potassium and potassium ion batteries. The new electrolyte is based on an organic phosphate which is effective even at the relatively low concentration levels necessary for mass production processes.
Flame retardant solvent
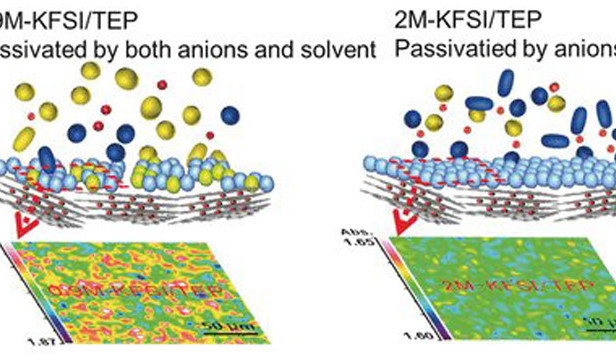
The active component of the electrolyte is triethyl phosphate which is known as a flame retardant. This material has already been tested for use with lithium-ion batteries, but very high concentrations are necessary to ensure long-term stability. The researchers combined the phosphate solvent with potassium salt to produce an electrolyte that didn’t burn and allowed stable charge-cycling of the assembled battery. The electrolyte solution has a concentration as low as 0.9 to 2 moles per litre, which makes it more suitable for large-scale production.Are potassium ion batteries the future?
The breakthrough in the new battery design is the formation of a uniform and stable solid-electrolyte interphase layer. This layer only occurs with phosphate electrolytes and facilitates a high number of stable charging cycles of the battery. The new battery design made from materials that are more abundant promises to slash the costs of large-scale energy storage solutions.The research findings are published in the journal Angewandte Chemie.
