New Cathode Boosts Li-ion Performance
May 01, 2019
on
on
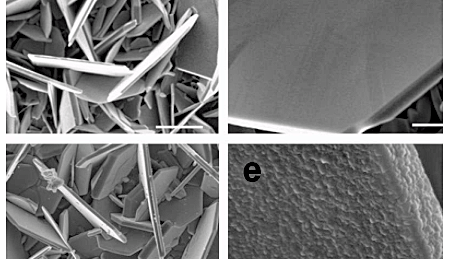
Researchers at the American RPI (Rensselaer Polytechnic Institute) have significantly improved the performance of lithium batteries using vanadium disulfide as a cathode material. They were able to massively optimize both capacity and fast charging behavior.
A lithium-ion battery charges and discharges as lithium ions move between two electrodes. In a conventional lithium-ion battery, the anode is graphite and the cathode is lithium cobalt oxide. In terms of price and other electrical properties, these materials are very good, which is why lithium-ion batteries continue to open up other applications. The researchers from Rensselaer, however, succeeded in further improvement with other materials.
The potential for VS2 has aroused interest in recent years, but until now the material instability has resulting in batteries with poor charge-cycle performance. The researchers at Rensselaer have not only found out why this instability occurs, but also developed a countermeasure.
The team found that the lithium insertion caused an asymmetry in the distance between the vanadium atoms known as the Peierls distortion. This is the cause of the decay of VS2 flakes. They developed a conformal nano-coating for these flakes using titanium disulphide (TiS2), a material that is immune to the Peierls distortion. This coating stabilizes the VS2 flakes mechanically and thus improves battery durability.
After solving this problem, the VS2-TiS2 electrodes also allowed a very high specific capacitance. Vanadium and sulfur allow high capacity and energy density due to their small size and light weight, which contribute to the production of compact batteries. In addition, it was shown that the capacity reduction which occurs after many charge cycles was not as pronounced as with other electrode materials, because VS2 / TiS2 is electrically conductive in contrast to cobalt oxide.
The research results were recently published in the Nature Communications journal.
A lithium-ion battery charges and discharges as lithium ions move between two electrodes. In a conventional lithium-ion battery, the anode is graphite and the cathode is lithium cobalt oxide. In terms of price and other electrical properties, these materials are very good, which is why lithium-ion batteries continue to open up other applications. The researchers from Rensselaer, however, succeeded in further improvement with other materials.
Vanadium disulfide
Nikhil Koratkar is one of the world's most cited researchers in this field, and his team made further improvements by replacing cobalt oxide with vanadium disulfide (VS2). The result is a battery with higher energy density, as VS2 is light. Thanks to its better conductivity, it also offers improved fast charging capability.The potential for VS2 has aroused interest in recent years, but until now the material instability has resulting in batteries with poor charge-cycle performance. The researchers at Rensselaer have not only found out why this instability occurs, but also developed a countermeasure.
The team found that the lithium insertion caused an asymmetry in the distance between the vanadium atoms known as the Peierls distortion. This is the cause of the decay of VS2 flakes. They developed a conformal nano-coating for these flakes using titanium disulphide (TiS2), a material that is immune to the Peierls distortion. This coating stabilizes the VS2 flakes mechanically and thus improves battery durability.
After solving this problem, the VS2-TiS2 electrodes also allowed a very high specific capacitance. Vanadium and sulfur allow high capacity and energy density due to their small size and light weight, which contribute to the production of compact batteries. In addition, it was shown that the capacity reduction which occurs after many charge cycles was not as pronounced as with other electrode materials, because VS2 / TiS2 is electrically conductive in contrast to cobalt oxide.
The research results were recently published in the Nature Communications journal.
Read full article
Hide full article


Discussion (0 comments)