Discreetly Discrete: On the Quantum Nature of Atoms
June 15, 2015
on
on
This article originally appeared in Elektor.POST #119 on April 24, 2015. Don't miss any updates, take out a free membership to Elektor.POST.
Nowadays, when reading through research papers it seems that concepts like quantum computers, valleytronics, or qubits are all the rage. Quite possibly they will soon be part of our everyday world. But hey! It’s taken a ton of important steps to get to our present level of understanding of the seemingly bizarre world of the atom. Let's celebrate just one of those steps.
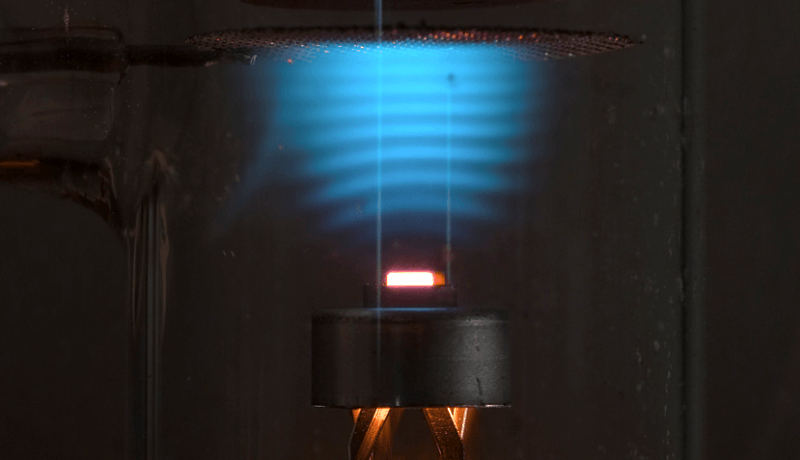
On a day like today, in fact exactly 101 years ago, on April 24, 1914, physicists James Franck and Gustav Ludwig Hertz—the nephew of Heinrich Hertz no less!—published their famous paper in the Deutsche Physikalische Gesellschaft (German Physical Society) demonstrating the quantum nature of atoms. This paper gave weight to the theory proposed by Niels Bohr just one year before that electrons could only occupy certain bound, discrete energy levels within the atom. They used a type of vacuum tube called a mercury vapor triode, in which it's possible to control the potential of all three electrodes and as its name implies, is filled with mercury vapor.
The experiment showed that as the (cathode-grid) accelerating voltage is gradually increased, current flow at the anode increases until the gate voltage reaches 4.9 V whereupon anode current suddenly drops to near zero. Increasing the gate voltage further produces the same anode current behavior at multiples of 4.9 V. A plot of the anode current looks like a rising sawtooth pattern as the gate voltage is linearly increased. The findings showed that electrons occupy discrete energy levels that can absorb or emit energy in only certain quantized amounts. Since then the phenomenon has been demonstrated using other elements as well. So yes, nature seems to work “quantized”.
It took more than 11 years until Franck and Hertz were finally awarded the Nobel Prize in Physics. Not surprising: The wheels of bureaucracy grind even slower than the ones in science.
Picture: © Ed Lochocki | Physics Soup
Nowadays, when reading through research papers it seems that concepts like quantum computers, valleytronics, or qubits are all the rage. Quite possibly they will soon be part of our everyday world. But hey! It’s taken a ton of important steps to get to our present level of understanding of the seemingly bizarre world of the atom. Let's celebrate just one of those steps.
On a day like today, in fact exactly 101 years ago, on April 24, 1914, physicists James Franck and Gustav Ludwig Hertz—the nephew of Heinrich Hertz no less!—published their famous paper in the Deutsche Physikalische Gesellschaft (German Physical Society) demonstrating the quantum nature of atoms. This paper gave weight to the theory proposed by Niels Bohr just one year before that electrons could only occupy certain bound, discrete energy levels within the atom. They used a type of vacuum tube called a mercury vapor triode, in which it's possible to control the potential of all three electrodes and as its name implies, is filled with mercury vapor.
The experiment showed that as the (cathode-grid) accelerating voltage is gradually increased, current flow at the anode increases until the gate voltage reaches 4.9 V whereupon anode current suddenly drops to near zero. Increasing the gate voltage further produces the same anode current behavior at multiples of 4.9 V. A plot of the anode current looks like a rising sawtooth pattern as the gate voltage is linearly increased. The findings showed that electrons occupy discrete energy levels that can absorb or emit energy in only certain quantized amounts. Since then the phenomenon has been demonstrated using other elements as well. So yes, nature seems to work “quantized”.
It took more than 11 years until Franck and Hertz were finally awarded the Nobel Prize in Physics. Not surprising: The wheels of bureaucracy grind even slower than the ones in science.
Picture: © Ed Lochocki | Physics Soup
Read full article
Hide full article



Discussion (0 comments)