Boron arsenide boasts high thermal conductivity
July 12, 2018
on
on
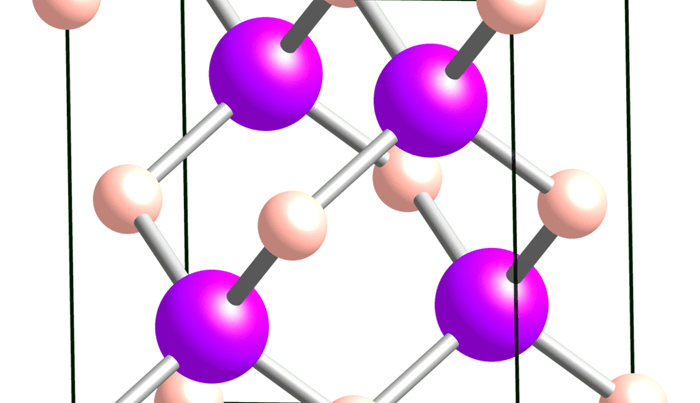
If it were to occur in nature it had been predicted that the cubic crystal structure of boron arsenide would give this material the property of high thermal conductivity. Researchers at the University of Illinois at Urbana-Champaign and the University of Texas, Dallas are now in a position to test this hypothesis because they have succeeded in building this crystal structure.
The crystals were synthesized using a process called chemical vapor transport. Here elemental boron and arsenic are combined in a vapor phase, then cooled to condense into small crystals. Trial-and-error synthesis has revealed the conditions under which crystals of sufficient quality are formed. The result is a material with a thermal conductivity of 1,000 W / m / K at room temperature – that’s twice as good as silicon carbide or silver and almost half as good as diamond itself.
Researchers are testing the material for use in heat-spreader applications – this is where tiny blocks of material with high thermal conductivity are directly in contact at points of high energy dissipation in a semiconductor chip such as a high power LED or processor. The blocks then form a thermal bridge to a much larger aluminum or copper heat sink. Without the highly conductive heat-spreaders, the small contact area between the heat source and heat sink would create a bottleneck in the heat dissipation pathway and reduce its performance.
Diamond is occasionally used to provide this heat-spreader function in more demanding applications but diamond is expensive and man-made versions suffer from structural defects, making them less efficient at the task. This new material could provide a better, cheaper alternative.
The crystals were synthesized using a process called chemical vapor transport. Here elemental boron and arsenic are combined in a vapor phase, then cooled to condense into small crystals. Trial-and-error synthesis has revealed the conditions under which crystals of sufficient quality are formed. The result is a material with a thermal conductivity of 1,000 W / m / K at room temperature – that’s twice as good as silicon carbide or silver and almost half as good as diamond itself.
Researchers are testing the material for use in heat-spreader applications – this is where tiny blocks of material with high thermal conductivity are directly in contact at points of high energy dissipation in a semiconductor chip such as a high power LED or processor. The blocks then form a thermal bridge to a much larger aluminum or copper heat sink. Without the highly conductive heat-spreaders, the small contact area between the heat source and heat sink would create a bottleneck in the heat dissipation pathway and reduce its performance.
Diamond is occasionally used to provide this heat-spreader function in more demanding applications but diamond is expensive and man-made versions suffer from structural defects, making them less efficient at the task. This new material could provide a better, cheaper alternative.
Read full article
Hide full article


Discussion (0 comments)