Graphite Boosts Battery Life
on
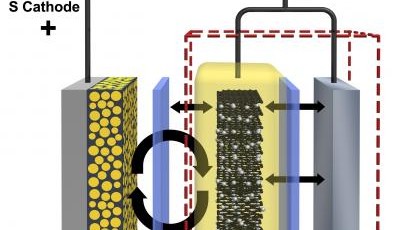
The weak link in electric vehicle technology is the method of energy storage and renewal, making the vehicles impractical for long distance use. The majority of today's electric vehicles use rechargeable lithium-ion batteries which still have a relatively poor energy density compared to conventional fossil fuels and require lengthy recharge cycles. A promising alternative battery chemistry is the lithium-sulfur battery. It can store as much as four times more energy per mass than lithium-ion batteries.
Unfortunately reactions at the battery’s sulfur-containing cathode form molecules called polysulfides that dissolve into the battery’s electrolyte. The dissolved sulfur eventually develops into a thin film called a solid-state electrolyte interface layer which coats the lithium-containing anode making the battery unusable after only 100 charge/discharge cycles.
Researchers at the US Department of Energy’s Pacific Northwest National Laboratory have succeeded in quadrupling the useful number of charge/discharge cycles. They have developed a graphite shield which moves the sulfur side reactions away from the anode's lithium surface, preventing it from growing the debilitating interference layer. The new hybrid anode combines graphite from lithium-ion batteries with lithium from conventional lithium-sulfur batteries.



Discussion (0 comments)